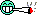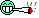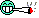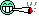hello guys,
troubles with users in forum were really not in the best time.
i have problems with these two plants, one is only clawed up as you can see, the other one is in flowering, and i thought it was light burn, but i don't think so anymore, can't figure out what's the problem.
picture 1 - i have this burned leaf maybe someone knows what this can be?
picture 2 - clawed up leafs, my temperature in room is fine, and humidity either, it's not the climate, because one other plant is doing really fine, just had some magnesium deficiency.
picture 3 and 4 - that's what i thought was light burn, but it's not, can it be nutrient burn ?
please help me if you can, thanks a lot in advance.
ps. i gave em some calcium nitrate and magnesium some time ago, along with my biobizz nutes, i've been using fish mix, bloom and grow now.
troubles with users in forum were really not in the best time.
i have problems with these two plants, one is only clawed up as you can see, the other one is in flowering, and i thought it was light burn, but i don't think so anymore, can't figure out what's the problem.
picture 1 - i have this burned leaf maybe someone knows what this can be?
picture 2 - clawed up leafs, my temperature in room is fine, and humidity either, it's not the climate, because one other plant is doing really fine, just had some magnesium deficiency.
picture 3 and 4 - that's what i thought was light burn, but it's not, can it be nutrient burn ?
please help me if you can, thanks a lot in advance.
ps. i gave em some calcium nitrate and magnesium some time ago, along with my biobizz nutes, i've been using fish mix, bloom and grow now.

 Have you had a look under the leaves where the rust spots are.? I dont think it could be, but do you have any mites eating in that area.. First thing definitely is to check the soil ph if you havent already. Hopefully someone with alittle more knowledge will chime in for you. Much Luck and hope she gets to better health. Stay positive about her.
Have you had a look under the leaves where the rust spots are.? I dont think it could be, but do you have any mites eating in that area.. First thing definitely is to check the soil ph if you havent already. Hopefully someone with alittle more knowledge will chime in for you. Much Luck and hope she gets to better health. Stay positive about her.