- Joined
- Jan 2, 2011
- Messages
- 10,876
- Reputation
- 1,990
- Reaction score
- 6,776
- Points
- 0
- Website
- www.youtube.com
THREAD LOCKED UNTIL COMPLETION
Meters JM uses and endorses:
View attachment Hannah 98129_98130.pdf
View attachment Accurate pH 8.pdf
This will be the full ph manual. Muddy and I have agreed to work in conjunction to make a more complete, in one place manual for PH, also known as Potential Hydrogen. So whether you're a noob or a pro... you'll probably catch a tip or 2 here.

This thread will be closed while its under construction:
To Start (excerpt from: https://www.autoflower.org/f7...s-list-11.html ) :
I have been doing a lot of research on sick plants and also helping out others a lot on sick plants!
Most of the stuff I have learned is from others and keeping up to date on there problems they are having. Some I have learned on my own, the sick plant troubles I have had was ph troubles! I didn’t really think the importance of how ph plays a role with your plants being healthy or dead.
I honestly think one of the most important parts of your growing is having a good solid ph tester, a digital one is the best to have. There are other ones you can buy as well, liquid ph test kits are inexpensive and get the job done if you can't afford a digital ph meter, STAY AWAY FROM SOIL TESTERS, they don't do the job and are not very accurate at all. If you get a soil tester, get a good one.. Accurate 8 makes a good one... (get the 12" probe version: )Ph test strips work well, even if you are on a budget! So if you rely on a soil tester and its tell you your soil is 7 and your having problems, 9 times out of 10 it's going to be your water ph that is messing up the soil ph check the water you are using. Unless you are using additives in your soil mixture like blood bone meal, and Peat moss those will throw your ph off too.
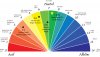
Adding nutes to your water can cause the ph to get low as well, so its best to test your ph of your water before and after you add your nutes. Nutrient deficiencies are mostly caused by human mistakes,along with to much or to little of the amount of nutrients available. The best range for nutrients to be absorbed is between a pH of 5 and 7 (ideally 6.3 - 6.5) and a (TDS) range of 800 to 3000 PPM.
Having these conditions will help making nutrient deficiencies a lot easier to overcome.
Charts:
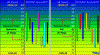





View attachment 135206
View attachment 135205

Example of Good ph on a HighRise Seeds Island Sweet Skunk:
View attachment 135208
View attachment 135207
not much dif... but...
Chart again:

Diagnostic Chart:

other self-diagnostic threads:
https://www.autoflower.org/f7/self-diagnose-your-plants-basic-deficiencies-list-11.html


Meters JM uses and endorses:
View attachment Hannah 98129_98130.pdf
View attachment Accurate pH 8.pdf
This will be the full ph manual. Muddy and I have agreed to work in conjunction to make a more complete, in one place manual for PH, also known as Potential Hydrogen. So whether you're a noob or a pro... you'll probably catch a tip or 2 here.

This thread will be closed while its under construction:
To Start (excerpt from: https://www.autoflower.org/f7...s-list-11.html ) :
I have been doing a lot of research on sick plants and also helping out others a lot on sick plants!
Most of the stuff I have learned is from others and keeping up to date on there problems they are having. Some I have learned on my own, the sick plant troubles I have had was ph troubles! I didn’t really think the importance of how ph plays a role with your plants being healthy or dead.
I honestly think one of the most important parts of your growing is having a good solid ph tester, a digital one is the best to have. There are other ones you can buy as well, liquid ph test kits are inexpensive and get the job done if you can't afford a digital ph meter, STAY AWAY FROM SOIL TESTERS, they don't do the job and are not very accurate at all. If you get a soil tester, get a good one.. Accurate 8 makes a good one... (get the 12" probe version: )Ph test strips work well, even if you are on a budget! So if you rely on a soil tester and its tell you your soil is 7 and your having problems, 9 times out of 10 it's going to be your water ph that is messing up the soil ph check the water you are using. Unless you are using additives in your soil mixture like blood bone meal, and Peat moss those will throw your ph off too.
Adding nutes to your water can cause the ph to get low as well, so its best to test your ph of your water before and after you add your nutes. Nutrient deficiencies are mostly caused by human mistakes,along with to much or to little of the amount of nutrients available. The best range for nutrients to be absorbed is between a pH of 5 and 7 (ideally 6.3 - 6.5) and a (TDS) range of 800 to 3000 PPM.
Having these conditions will help making nutrient deficiencies a lot easier to overcome.
Charts:






Now on to optimal PH:
They say a picture is worth a thousand words and a symbol worth a thousand pictures:
How about just 2 graph pictures? I think its congruent!


So now the picture on PH is probably coming together for you if you weren't already familar.
Example of PH being out on a flowering Auto Cinderella 99:They say a picture is worth a thousand words and a symbol worth a thousand pictures:
How about just 2 graph pictures? I think its congruent!


So now the picture on PH is probably coming together for you if you weren't already familar.
View attachment 135206
View attachment 135205

Example of Good ph on a HighRise Seeds Island Sweet Skunk:
View attachment 135208
View attachment 135207
not much dif... but...
Chart again:

Diagnostic Chart:

other self-diagnostic threads:
https://www.autoflower.org/f7/self-diagnose-your-plants-basic-deficiencies-list-11.html


Last edited: