- Joined
- Feb 13, 2018
- Messages
- 2,712
- Reputation
- 0
- Reaction score
- 11,547
- Points
- 0
- Currently Smoking
- granddy purp
That's about 4-5g per gal niceI'm now down to just SC and BE @ 1/2 tsp per 5 gallon no Cal Mag stopped that when stopped MC
That's about 4-5g per gal niceI'm now down to just SC and BE @ 1/2 tsp per 5 gallon no Cal Mag stopped that when stopped MC
Not per gal, that's 4-5 g per 5 galThat's about 4-5g per gal nice
I'm now down to just SC and BE @ 1/2 tsp per 5 gallon no Cal Mag stopped that when stopped MC
No, I remember I down feed until I feel I'm around 10 days ,sometimes longer sometimes less, b4 harvest letting the plant use the nutrients that are remaining in the soilno kidding. that is wild. Is that for all of bloom you're doing this?

My first bag was a very fine powder that dissolved easily this pack is bigger more granular so I crush it up and make it more powdery and it should dissolve easierThis was likely already covered but I can’t find it... are you guys finding that bud explosion does not dissolve nearly as easily as megacrop does? I added it for the first time last night and even though I start in a jar of warm water to shake it all up I was having a hell of a time getting it to dissolve
I’m just going to tell you my experience and you can take it for what it is. I’m always amazed at the differences folks have with nutrients. I have never used warm water, although I know some recommend it. I normally use cool rainwater, which I move around in blue five gallon water containers like these.This was likely already covered but I can’t find it... are you guys finding that bud explosion does not dissolve nearly as easily as megacrop does? I added it for the first time last night and even though I start in a jar of warm water to shake it all up I was having a hell of a time getting it to dissolve
 Then I add MegaCrop products directly to the container and then mix it up. Never had any issues with it not mixing or dissolving. Drops my waters’ pH to about 5.5, I pH up to 6.5 and allow to sit for 24 hours, check the pH again, and add to the reservoir. It normally stays within 0.10 of what I start with. The tendency is to creep up very slowly and usually have to replace it before adjusting the pH again.
Then I add MegaCrop products directly to the container and then mix it up. Never had any issues with it not mixing or dissolving. Drops my waters’ pH to about 5.5, I pH up to 6.5 and allow to sit for 24 hours, check the pH again, and add to the reservoir. It normally stays within 0.10 of what I start with. The tendency is to creep up very slowly and usually have to replace it before adjusting the pH again.This was likely already covered but I can’t find it... are you guys finding that bud explosion does not dissolve nearly as easily as megacrop does? I added it for the first time last night and even though I start in a jar of warm water to shake it all up I was having a hell of a time getting it to dissolve
Then I add MegaCrop products directly to the container and then mix it up. Never had any issues with it not mixing or dissolving
 --- yeah, MC goes right into solution easily, but then it's a complex mix of things, including different nutrient element salts than what's in BE,..... I've noticed my self, and from a few others this more difficult to dissolve, and that's because of what exactly it's made of (2 salts, that's it)... not all salts dissolve as readily as others, part of the normal chemistry there!
--- yeah, MC goes right into solution easily, but then it's a complex mix of things, including different nutrient element salts than what's in BE,..... I've noticed my self, and from a few others this more difficult to dissolve, and that's because of what exactly it's made of (2 salts, that's it)... not all salts dissolve as readily as others, part of the normal chemistry there!Yeah, what he said lol. Breaking it down bud, nice. Who says people who smoke bud aren't smart. Would it dissolve better if you mix BE 1st, or does order mater? I was reading something about potassium deficiency and the article mentioned that it's better if you put your calmag in water 1st. Not sure if true, but why risk it.--- yeah, MC goes right into solution easily, but then it's a complex mix of things, including different nutrient element salts than what's in BE,..... I've noticed my self, and from a few others this more difficult to dissolve, and that's because of what exactly it's made of (2 salts, that's it)... not all salts dissolve as readily as others, part of the normal chemistry there!
Keep this in mind for a definition of what a "salt" is: In chemistry, a salt is an ionic compound which is made up of two groups of oppositely charged ions. The ion with a positive charge is called a cation, and the one with a negative charge is called an anion. How many of each type of ion the salt has is important because the compound must have an overall electrical charge of zero - that is, an equal balance between positive charge and negative charge.
 uhhhhh,...
uhhhhh,...  ... no mate, this is about the thermodynamics of a salt dissolving --(think relative ease of water pulling off component ions)... technical stuff, but it comes down to charge strengths and the specifics of the reaction in water,... this could go into a whole lecture, particularly about water! The reason water can do this with both + and - charges is this: the water molecule itself is net neutral in charge, but has very strongly polar +/- ends...
... no mate, this is about the thermodynamics of a salt dissolving --(think relative ease of water pulling off component ions)... technical stuff, but it comes down to charge strengths and the specifics of the reaction in water,... this could go into a whole lecture, particularly about water! The reason water can do this with both + and - charges is this: the water molecule itself is net neutral in charge, but has very strongly polar +/- ends... 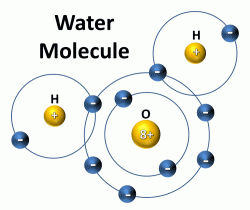
 quiz Friday!!
quiz Friday!! 
